To help protect your child against certain HPV-related cancers and diseases, complete the GARDASIL 9 vaccine series
The CDC notes that HPV vaccination may begin at age 9, and recommends it routinely for the 11- to 12-year-old age group.
See the dosing schedule below and talk to your child’s doctor about when your child should start.
Make sure your child completes their recommended vaccine schedule.
The number of recommended doses is based on age when the first dose is given.
If your child is 9- to 14-years-old, your child’s doctor will determine whether your child needs a 2-dose or 3-dose schedule of GARDASIL 9.
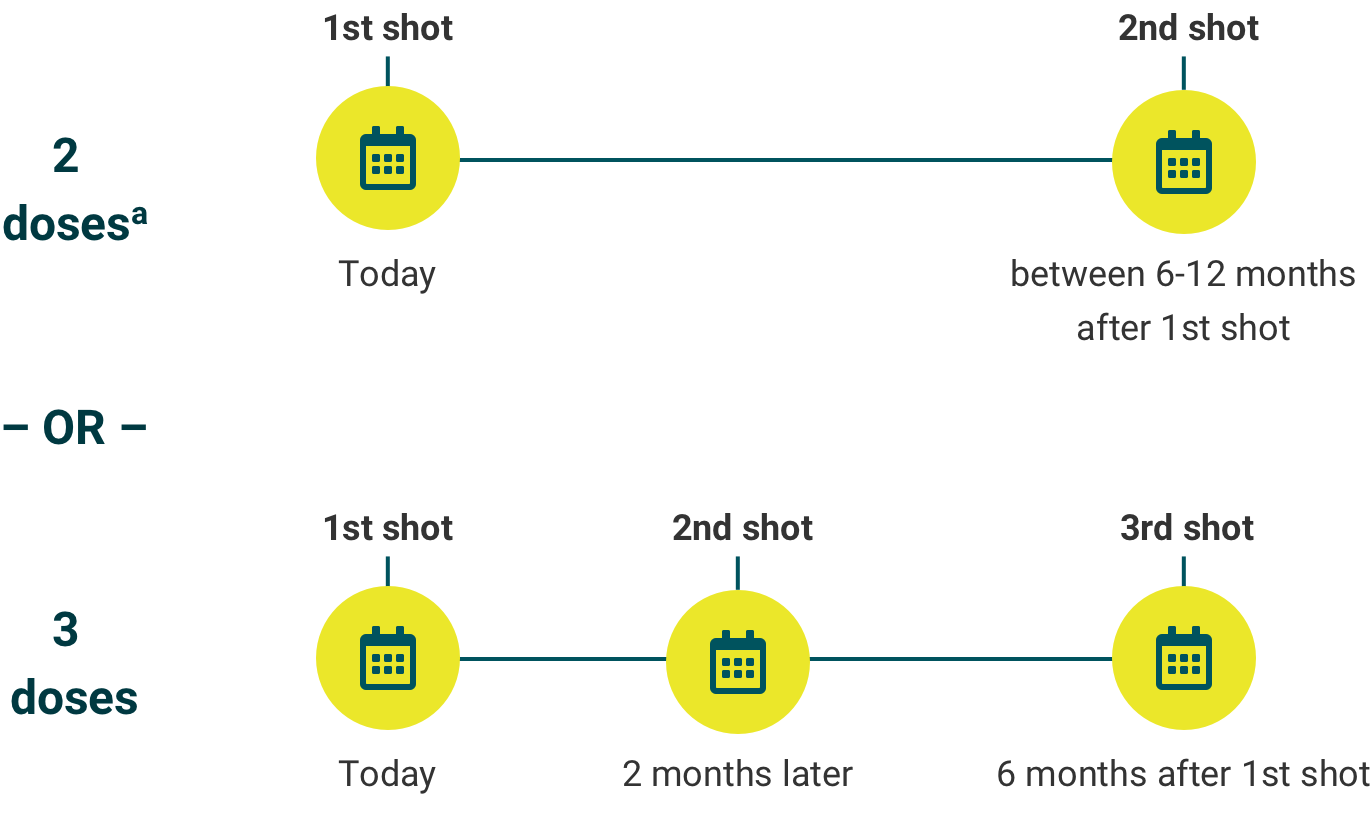
aIf the second shot is given earlier than 5 months after the first shot, your child will need to get a third shot at least 4 months after the second shot was given.
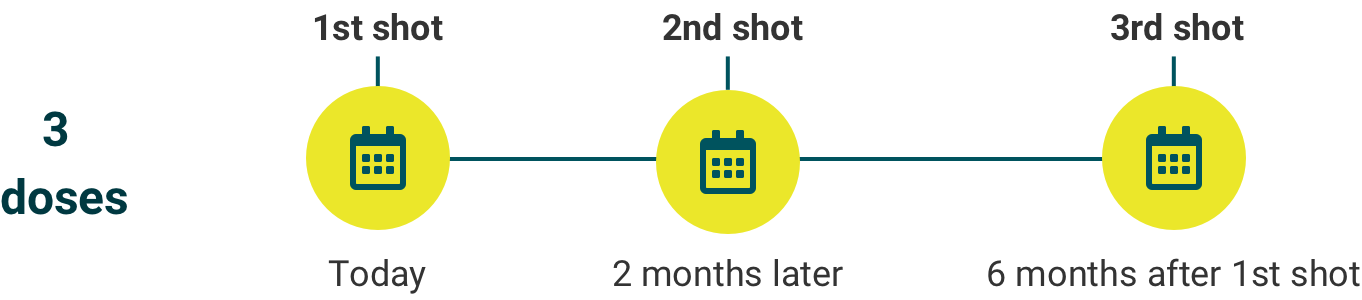
For individuals 15- to 45-years-old
Fainting can happen after getting an HPV vaccine. Sometimes people who faint can fall and hurt themselves. For this reason, your child’s doctor may ask your child to sit or lie down for 15 minutes after he or she gets GARDASIL 9. Some people who faint might shake or become stiff, which may need treatment.
If your child misses a dose of GARDASIL 9
Make sure that your child gets all doses recommended by your health care professional so that your child gets the best protection.
If your child misses a dose, tell their health care professional and they will decide when to give the missed dose.
To help avoid a missed dose of GARDASIL 9, make an appointment for your child’s next dose before leaving the doctor’s office.

Some questions parents may have about GARDASIL 9
What are the ingredients of GARDASIL 9?
The ingredients are proteins of HPV Types 6, 11, 16, 18, 31, 33, 45, 52, and 58, amorphous aluminum hydroxyphosphate sulfate, yeast protein, sodium chloride, L-histidine, polysorbate 80, sodium borate, and water.
For more information on GARDASIL 9, talk to your child’s doctor.
Can my child get GARDASIL 9 with other vaccines?
Yes. Your child may get GARDASIL 9 at the same time as:
- Menactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine]a
- Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine Adsorbed (Tdap)]a
Studies show that when GARDASIL 9 was given at the same time as Menactra and Adacel, there was more swelling in the location of the shot.
aMenactra [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] and Adacel [Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine Adsorbed (Tdap)] are the trademarks of their respective owners and are not trademarks of Merck Sharp & Dohme LLC.